The DGS specifically sought clarification on the clinical benefit (SMR) distinction across trimesters, the role of valaciclovir, and identification of specific at-risk sub-populations (e.g., young mothers with first young child).
Context and Disease Burden
CMV is a common DNA virus, with humans as its reservoir, transmitted through various bodily fluids. While often asymptomatic or causing mild symptoms in infected individuals, primary CMV infection (PIM) acquired in utero can lead to severe sequelae in newborns, including hearing impairment and neurodevelopmental delays. In France, these severe complications affect approximately 1 to 6 newborns per 100,000 births annually. The risk of vertical transmission is estimated at 21% during the periconceptional period and 36.8% in the first trimester. About 90% of newborns with congenital CMV (cCMV) are asymptomatic at birth, but 10% may develop sequelae, mainly hearing loss, within the first six years.
Historically, French public health recommendations, from ANAES (2004) to HCSP (2018, 2023), did not recommend systematic CMV screening during pregnancy. This stance was based on the lack of proven efficacy of antiviral treatments to prevent mother-to-fetus transmission or sequelae, and an unfavorable benefit-risk ratio. However, evolving scientific knowledge, increasing clinical practice diffusion of screening and treatment, and societal expectations prompted this re-evaluation.
Therapeutic and Screening Landscape
Currently, no antiviral medication holds marketing authorization (AMM) specifically for preventing PIM during pregnancy. Despite this, valaciclovir is increasingly used in clinical practice for this indication. The French Centre de Référence sur les Agents Tératogènes (CRAT) notes that extensive data exist on valaciclovir use in the second and third trimesters for curative treatment, and no teratogenic effect has been identified for first-trimester exposure, regardless of dosage. High-dose valaciclovir (8 g/day) necessitates adequate hydration and monitoring of maternal renal function to prevent acute kidney injury, which can be mitigated by splitting the daily dose into four administrations.
Internationally, systematic CMV screening for pregnant women does not have a global consensus. As of June 2025, Italy, Greece, and Israel are the only countries that recommend systematic screening with subsequent valaciclovir treatment for positive cases. Other countries, such as Australia, Canada, the United Kingdom, and Germany, either propose targeted screening or do not recommend systematic screening. The lack of consensus often stems from persistent uncertainties regarding valaciclovir’s efficacy in preventing fetal transmission and the long-term safety of the fetus.
HAS Evaluation Methodology
The HAS’s evaluation employed a multidimensional approach:
- Analysis of the HCSP 2023 report: Identified areas needing updating, especially on valaciclovir efficacy and ethical considerations.
- International recommendations panorama: Reviewed global screening practices.
- Diagnostic test performance: Evaluated the reliability of serological tests (IgG, IgM, avidity).
- Efficacy of preventive hygiene measures: Assessed interventions to prevent maternal PIM.
- Re-evaluation of valaciclovir efficacy and safety: Focused on studies published after 2020, including a meta-analysis using a sophisticated hierarchical normal-normal model (NNHM) to combine randomized and observational data. This model aims to rigorously integrate external data, providing a robust estimate. Maternal safety data was also collected from national databases (BDM, CRAT).
- Economic evaluation: Assessed cost-effectiveness of screening and treatment strategies based on recent studies.
- Analysis of French practices: Utilized national health data (SNDS) for 2022-2023 to map CMV serology testing and valaciclovir prescription patterns.
- Ethical analysis: Explored ethical considerations using the four principles of Beauchamp and Childress (beneficence, non-maleficence, autonomy, and justice).
- Stakeholder consultation: Engaged patient associations, professional bodies, and experts for their perspectives.
Key Findings of the Evaluation
- Valaciclovir Efficacy: While valaciclovir lacks AMM, HAS’s re-analysis, using a robust statistical method combining the Shahar-Nissan randomized trial with four observational studies, demonstrated a significant effect of valaciclovir (8g/day) in reducing vertical transmission of CMV (Odds Ratio between 0.33 and 0.39). This finding is considered robust. However, the long-term safety and effects on fetal/newborn complications (frequency and severity) remain uncertain and require large-scale, robust studies.
- Valaciclovir Safety: Data from 2007-2023 on approximately 970 treated pregnant women shows no signal of teratogenicity. Maternal tolerance is generally good, though high doses require monitoring for acute renal insufficiency, which is usually reversible.
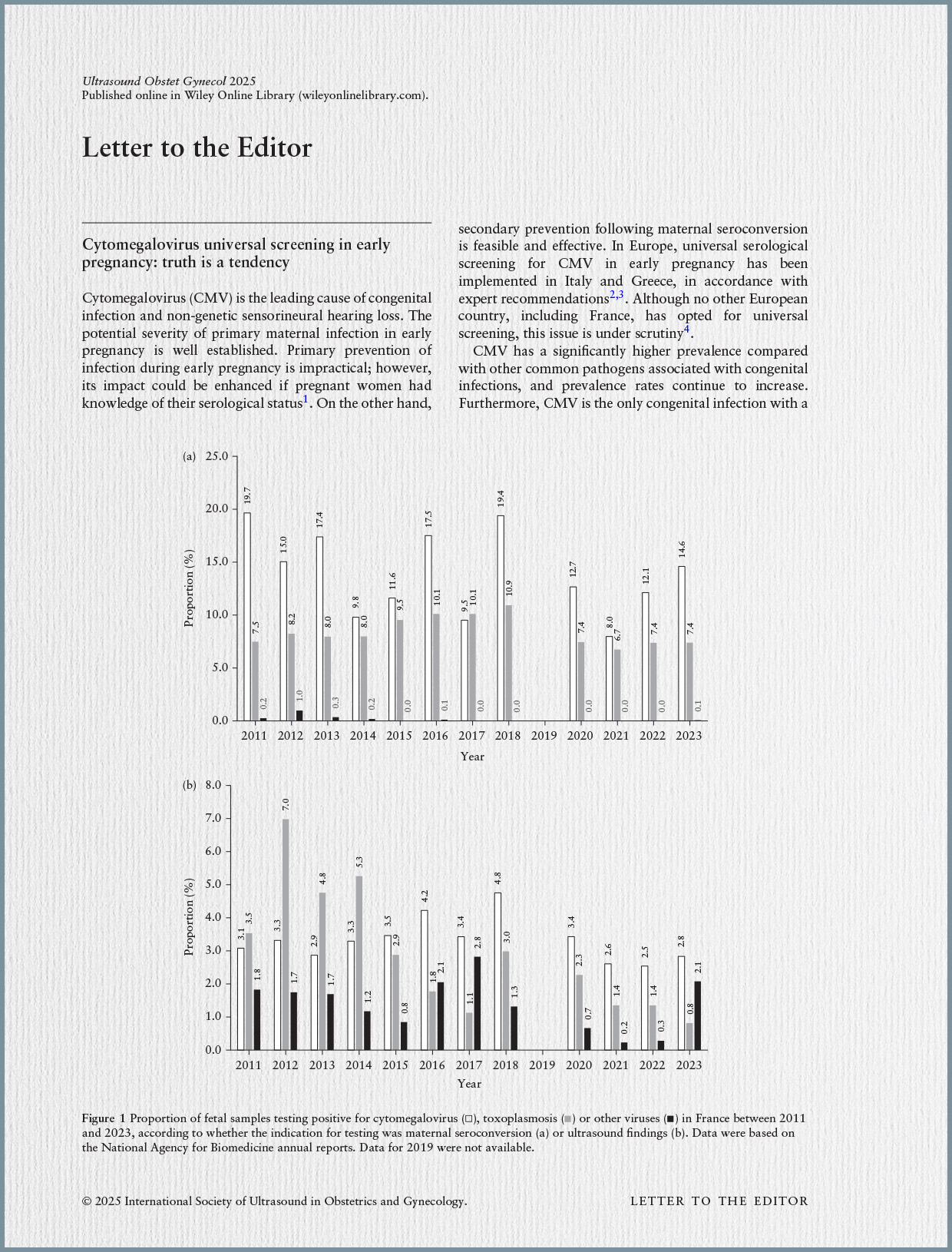
- French Practices: An analysis of SNDS data revealed a progressive increase and diffusion of valaciclovir prescriptions for CMV during pregnancy between 2022 and 2023, with 79% of initial prescriptions made by hospital physicians. There is significant regional heterogeneity, with higher rates in Île-de-France and Pays de la Loire. Approximately one-third of pregnancies are currently undergoing CMV screening in France. These practices are currently outside of regulated frameworks (AMM or compassionate use authorization).
- Medico-Economic Aspects: A recent French study (Perillaud-Dubois et al., 2023) suggests a benefit from systematic screening, but international studies are more nuanced. HAS noted that current models have limitations, such as not fully capturing the temporal dimension of events and the long-term costs of sequelae. While systematic screening might have significant initial costs, it could be economically relevant in the long term by reducing expenses related to congenital CMV complications.
- Ethical Considerations: The ethical analysis, based on Beauchamp and Childress’s principles, highlighted several issues. Key concerns include ensuring informed consent due to inherent uncertainties regarding the unpredictable outcomes of mother-to-fetus transmission and unknown long-term effects of valaciclovir. There is also a risk of “routinization” of screening, potentially leading to pregnant women delegating their autonomy to healthcare providers. The HAS emphasizes the need for transparent and comprehensive information to allow informed decision-making.
- Stakeholder Views: Patient associations were largely favorable to systematic screening, provided clear information is given and healthcare professionals are adequately trained. The Fondation pour l’audition, for instance, strongly advocated for screening due to CMV being a leading non-genetic cause of congenital deafness. Professional bodies, like the CNP-MIT, expressed that it seems reasonable to offer valaciclovir, but stressed the need for further research to address remaining uncertainties. Experts debated the HCSP’s previous conclusions, with some advocating strongly for systematic screening.
HAS Recommendation
Based on its comprehensive evaluation, the HAS made the following recommendation: The HAS recommends implementing a systematic national screening program for CMV infection for all pregnant women whose serological status is negative or unknown. This measure will be re-evaluated after three years of implementation to assess its relevance and consider its continuation.
The HAS also emphasizes the need for further studies to address persistent uncertainties concerning:
- National epidemiological data (maternal seroprevalence, frequency, and severity of neonatal and childhood complications).
- Long-term safety of valaciclovir on a larger scale.
- The effect of valaciclovir on fetal/newborn infections (frequency and severity of complications).
- The overall performance of the test sequence (IgG, IgM, IgG avidity).
The recommendation also acknowledges the expected increase in amniocenteses and associated risks following systematic screening. It underscores the importance of transparent and comprehensive information for pregnant women regarding the benefits, risks, and remaining uncertainties of screening.