Background and Clinical Context
Chronic histiocytic intervillositis (CHI) is an uncommon placental inflammatory disorder that has long posed diagnostic and therapeutic challenges for clinicians. Prior to the publication of this review, the condition was primarily described in pathology literature, with limited integration into internal medicine, obstetrics, and multidisciplinary clinical care. Mekinian and colleagues sought to address this gap by providing a comprehensive synthesis of existing knowledge on CHI, with particular emphasis on clinical presentation, histological diagnosis, recurrence risk, associated diseases, and management strategies.
This article occupies a pivotal position within the knowledge base because it bridges placental pathology and clinical medicine. Unlike purely pathological studies, it frames CHI as a systemic obstetrical disorder with immunological implications and positions internists—especially those managing autoimmune diseases—as key contributors to patient care.
Objectives
The objectives of this narrative review were:
- To summarize current knowledge regarding the definition and histological diagnosis of chronic histiocytic intervillositis
- To describe clinical manifestations and obstetrical outcomes associated with CHI
- To review data on recurrence rates and prognostic factors
- To explore associated autoimmune and inflammatory diseases
- To discuss current, albeit non-standardized, therapeutic management strategies
Definition and Histopathological Criteria
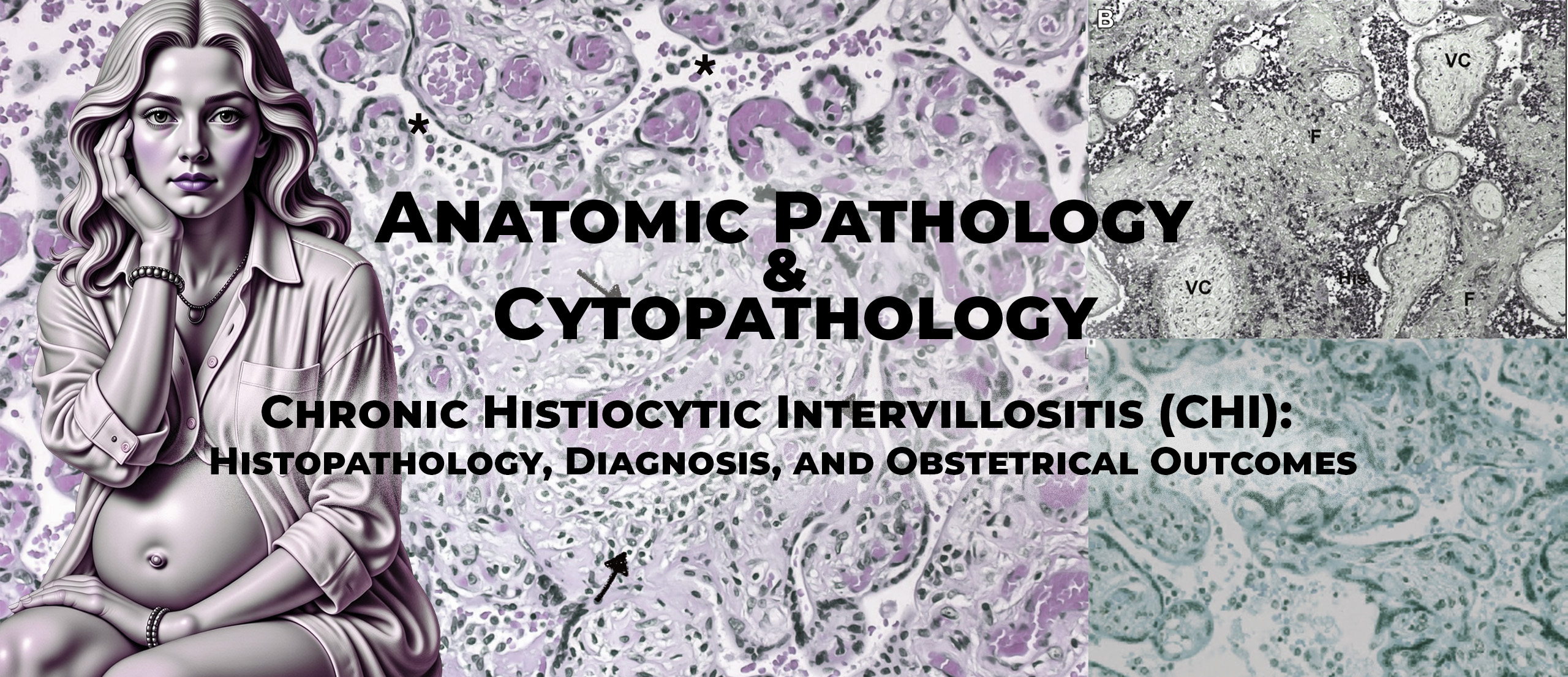
The authors define CHI as a placental lesion characterized by maternal histiocytic infiltration of the intervillous space, often accompanied by fibrinoid deposits. These macrophages are of maternal origin and are typically identified by CD68 immunostaining.
A major issue highlighted is the lack of standardized histological criteria. Several unresolved questions remain, including:
- Whether immunohistochemistry is mandatory for diagnosis
- How to quantify lesion extent reproducibly
- Whether C4d staining should be included in routine evaluation
The review summarizes different histological scoring systems proposed in the literature. These systems generally assess:
- Extent of intervillous involvement (focal, moderate, massive)
- Distribution (diffuse versus multifocal)
- Intensity of macrophage infiltration
- Presence and extent of fibrin deposition
Importantly, the authors emphasize that massive and diffuse CHI lesions are strongly associated with adverse outcomes, whereas limited lesions may occasionally be found in placentas from uncomplicated pregnancies.
Epidemiology and Frequency
CHI is described as a rare condition, with reported prevalence varying widely depending on the population studied. In unselected placental series, CHI is identified in approximately 0.3–0.5% of placentas. However, among placentas examined following fetal loss or severe growth restriction, prevalence may rise significantly, reaching up to 20% in selected cohorts.
The authors underline that the true incidence of CHI is likely underestimated due to:
- Limited awareness among clinicians
- Inconsistent placental examination practices
- Variability in histological diagnostic thresholds
Clinical Presentation and Obstetrical Outcomes
From a clinical standpoint, CHI is associated with a severe obstetrical phenotype. The most frequently reported complications include:
- Recurrent early miscarriages, often in the first trimester
- Intrauterine growth restriction (IUGR), frequently severe and early-onset
- Intrauterine fetal death (IUFD)
- Prematurity, typically secondary to placental insufficiency
The review highlights that IUGR is the most characteristic complication, reported in approximately 50% of affected pregnancies. Growth restriction is often severe (<3rd percentile) and may be associated with abnormal Doppler findings, although uterine Dopplers can remain normal in some cases, emphasizing the placental rather than vascular origin of pathology.
The authors also note that different adverse outcomes may occur in successive pregnancies in the same woman, reflecting variability in lesion timing and severity.
Recurrence Risk and Prognosis
One of the most clinically relevant aspects of CHI is its high recurrence rate. Across published series summarized in the review, recurrence rates range from 18% to over 70%, with an average around 30–40%.
Several important observations are emphasized:
- Recurrence risk is higher after severe or massive lesions
- Recurrent CHI often presents earlier in gestation than the index pregnancy
- Lesions may become progressively more severe, suggesting immunological memory
Despite these trends, the authors stress that no reliable predictive biomarker exists to identify women at highest risk of recurrence.
Interestingly, placental alkaline phosphatase elevation has been reported in some cases and may correlate with fibrin deposition and severe lesions, but this marker lacks specificity and validation.
Associated Diseases and Etiological Considerations
In most cases, no clear etiology is identified. However, Mekinian et al. emphasize the importance of a systematic etiological workup, particularly in cases of recurrent pregnancy loss.
Autoimmune Diseases
A significant proportion of women with CHI have associated autoimmune conditions. Reported associations include:
- Antiphospholipid syndrome
- Systemic lupus erythematosus
- Sjögren’s syndrome
- Autoimmune thyroid disease
- Celiac disease
In a prospective French cohort cited by the authors, approximately 29% of women with CHI had a documented autoimmune disease. This observation supports an immune-mediated pathogenesis and justifies involvement of internists and immunologists in patient management.
Infectious Causes
Although CHI is defined as a non-infectious lesion, certain infections—most notably placental malaria—can produce similar intervillous macrophage infiltrates. The authors stress the importance of excluding infections, including TORCH pathogens, particularly in endemic or high-risk settings.
Genetic Causes
In cases of recurrent early miscarriage, chromosomal abnormalities must also be excluded, as abnormal fetal karyotypes may coexist with CHI-like lesions.
Pathophysiological Hypotheses
The review strongly supports an alloimmune rejection hypothesis, in which maternal immune responses are directed against paternal antigens expressed by the placenta. Supporting arguments include:
- Predominance of maternal macrophages
- Presence of complement activation (C4d) in some cases
- Detection of anti-paternal HLA antibodies in affected women
- High recurrence rates resembling immune memory
The authors also discuss potential roles of innate immunity, including Toll-like receptor activation and inflammatory cytokine production, which may promote macrophage recruitment and adhesion to the syncytiotrophoblast.
Management and Therapeutic Strategies
A major contribution of this article is its discussion of management, despite the absence of randomized controlled trials. The authors clearly state that no treatment is currently validated, and all strategies are empirical.
Therapeutic approaches reported in the literature include:
- Low-dose aspirin
- Low-molecular-weight heparin
- Corticosteroids
- Hydroxychloroquine
- Combination regimens
These treatments are rationalized by the presumed immune-mediated pathophysiology and are particularly considered in women with:
- Recurrent CHI
- Severe obstetrical complications
- Associated autoimmune disease
The review emphasizes the need for multidisciplinary management, involving obstetricians, internists, pathologists, and immunologists.
Strengths and Limitations
Strengths
- Comprehensive synthesis of pathological and clinical data
- Integration of internal medicine perspective
- Clear identification of unmet clinical needs
Limitations
- Narrative, non-systematic review
- Reliance on heterogeneous retrospective studies
- Lack of therapeutic efficacy data
Educational Value
For teaching purposes, this article is essential because it:
- Frames CHI as a multisystem clinical problem, not just a pathological curiosity
- Highlights diagnostic uncertainty and real-world decision-making
- Provides a rationale for immunomodulatory therapies despite limited evidence
It sets the stage for later mechanistic studies that firmly establish CHI as a form of maternal–fetal alloimmune rejection.